|
|
|
|
|
|
|
|
|
|
|
Dry Suspension for
Film Coating
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Extended-release
System for Film Coating
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The system through
Pellet
|
|
|
|
|
|
|
|
|
|
Vicegerent Products
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Downlad the PDF DOC of our products to know all
details.
|
|
|

|
|

|
|
|
|
|
|
THINFILM®Normal Moisture Proof(TF-NMP)
|

|
|
This type is
suitable to the tablets that do not absorb vapor quickly such as
those completely or half contains raw plant medicinal medicine.
|
|
|
- Cutting off the
vapor more effectively
- Increasing the
stability and lengthens the storage life of the medicine.
- High mechanical
strength and adhesive of the film membrane
- Filming and coloring
quickly
|
|
|
|
Items
|
Hardness(Kg·N)
|
Breakage(%)
|
|
Tablets
|
4.50
|
0.51
|
|
Ordinarily coated tablets
|
7.23
|
0.31
|
|
TF-NMP coated tablets
|
11.8
|
0.04
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
THINFILM®Enhanced Moisture Proof(TF-EMP)
|

|
|
With the
characteristics of simple preparation, quick coloring and great
capability of moisture proof, This type is suitable to the
tablets that need to isolate the vapor strictly such as those
that completely contains concrete of TCM.
|
|
|
|
|
|
Unique formula
- Selecting high
polymer material of special moisture-proof performance and
matching with other many kinds of high polymers material
- Firmer film membrane
- Higher transmission
resistance of vapor
Isolation function
- Higher transmission
resistance of vapor that can protect the tablets from
breaking、inflation
or distortion.
- Increasing medicine
stability and lengthening the term of validity of medicines.
- During guarantee
stronger isolation of water vapor, the membrane keeps high
mechanical strength and adhesion, and dispersingh rapidly.
- The lake pigments
used cannot be influenced by the moisture absorption
ingredient of medicines and the color can maintain longer .
|
|
|
|

|
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
THINFILM®Instant Disintegration(TF-ID)
|

|
|
|
It is usually
used in oral solid dosage form that has the requirement of
disintegration.
|
|
|
- Protects the tablet
cores and colors
- Film membrane
disintegrate instantly
- Be suitable for the
dispersing tablet and buccal tablet of TCM
Unique formula
- Use special formula
design and advanced preparation process
- Film membrane
harmonizes with sense of the taste, touch and smell of the
tablet cores
Instant disintegration
- Increase
disintegration of the film membrane and the dissolution rate
of the API
- Dissolve instantly
when in mouth and have little feeling of membrane
Superior performance
- Increase the
stability and lengthen the storage life of the medicine
- Film and color
quickly
- High mechanical
strength and adhesive of the membrane
|
|
|
|
Items
|
TF-NMP
|
TF-ID
|
|
Buccal tablet
|
60-80s
|
30-50s
|
|
Dispersing tablet
|
100-120s
|
30-50 or no time delay
|
|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
THINFILM®Brightness&Luster Ferric Oxide(TF-BLFO)
|

|
|
|
Be suitable for
the oral solid dosage form that have high request of color cover.
|
|
|
- Strong cover ability、few
amount and higher color radiance.
- Take the ferric
oxide series as the color coating
- Ultra glare
brightness, exquisite smooth clothes membrane, and bright
and stable color
- Very big visual
impulse and allure to the consumer.
- Accelerate
disintegration and release compared to common product
|
|
|
|
Items
|
TF-NMP
|
TF-BLFO
|
|
increased weight of coating(%)
|
6
|
4.5
|
|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
THINFILM®Enteric System(TF-ES)
|

|
|
|
It can protect
gastric mucosal from influence of irritant medicine and protect the
medicines that are unstable in gastric juice, especially suitable
for the medicines that need to release in specific intestines
spot.
Taking HPMC、phthalate ester
and acrylic resin, adding the plasticizer, the color lake and
other kind of chemical additive, the film membrane has very
strong mechanical strength and dissolves depend on the pH changes
and guarantees medicine to release safely and effectively.
|
|
|
|
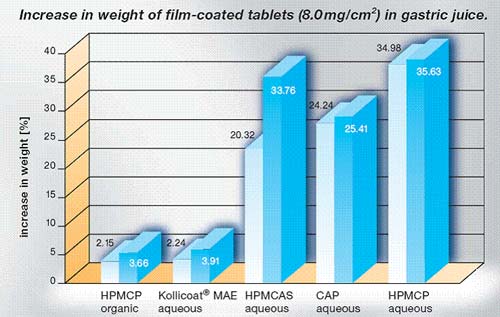
Ultra strong protection
- Protect medicine
from corroding of gastric juice, with only 4% increases;
- Dissolve rapidly
when pH elevates ;
- Reduce the poisonous
side effect of the medicine ;
- Provide the
protection in medicine preparation packing, transporting and
storing process.
Detention releases
- Have very strong
resistivity to the gastric juice and high bioavailability;
- The acid-proof
performance and the intestinal released performance is still
superior after long time;
- storing or under the
storing condition of high temperature or high humidity;
- The film membrane
affects release and absorption of the medicine slightly.
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
THINFILM®Ethylcellulose Aqueous Dispersion(TF-EAD)
|

|
|
|
It is an ethyl
cellulose coating system completely using water as dispersing medium
and used in oral solid extended release preparation. With the
characteristics of highly effective coating, simple operation and
no plastification, it can form an extended release coating
membrane, make the medicine keep releasing and maintain effective
vivo plasma concentration.
|
|
|
|
Unique Formula
- Take the ethyl
cellulose as a polymer, make the ethyl cellulose to micro
particles to dispersing in water and forming a kind of
emulsion.
- Good physical
stability.
- For the medicine
that is sensitive to acid and that have the property of
water affinity, it is your first choice.
Control Release
- Release not keen to
acid or alkalinity.
- Release obey to zero
and have good ruggedness.
- Control release
through the thickness of the film membrane.
Simple Operation
- Complete water
system, no organic solvent.
- Eliminate the
flammable explosive and toxic dangers of organic solvent.
- No special request
to the production equipment.
- With 30% solid
content but still lower viscosity, it reduces the operating
time and simultaneously avoids pot, bridging of tablets.
- Without usage of
plasticizer, the operation is more convenient.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
Extended-release Drug
Delivery System through Pellet
|

|
|
|
We provide
massive coating products of different functions and colors, as
well as entire dosage formulation.
|
|
|
|
Formula Design
- Based on customers’
requests, we provide the most detailed comprehensive formula
parameter to the skeleton pellet、the
membrane controlled pellet or both unified controlling
pellet.
- Improve the original
formula to achieve the best.
- Provide entire
formula design and the technical support from the laboratory
tries, experimental to the final industrialization
production
Film Coating
- Use different
coating materials to achieve different operation
requirements.
- Simple operation,
gorgeous appearance, low cost and stable quality.
Superior Performance
- It makes the
medicine distribute widely and evenly in the
gastro-intestinal tract, which enhances the bioavailability,
reduces or eliminate medicine stimulating to
gastro-intestinal tract.
- Not influenced by
food transportation rhythm or gastric evacuation.
- Rule of medicine
releases which cannot affect that of the entire preparation
seriously has good ruggedness compared to that of individual
pellet.
- To meet different
needs, pellets of different releasing rate can be filled in
capsule
- Different
compositions of compound capsule have a better stability and
less mutual function between different medicines.
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
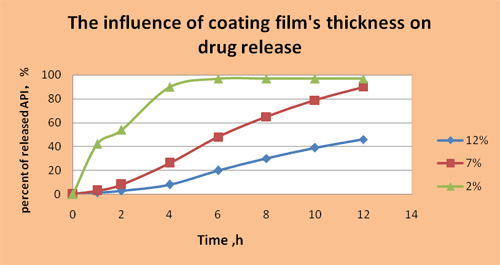
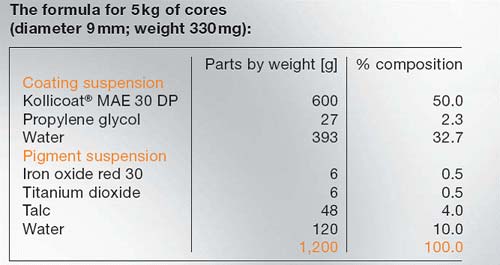
Kollicoat®MAE 30DP
|

|
|
|
Kollicoat® MAE 30
DP is marketed as an aqueous dispersion with a solids content of
30 %. The milky white, low viscosity product has a faint,
characteristic odour. The dispersion contains 0.7% sodium lauryl
sulfate (USP) and 2.3 % Polysorbate 80 as emulsifying agents.
|
|
|
|
Kollicoat® MAE applied in low coating levels,
0.5–2.0mg/cm2solids, can be used for the following purposes:
- Masking unpleasant
tastes and odours
- Protecting
incompatible active substances
- Protection against
atmospheric humidity (short-term)
Kollicoat® MAE applied in high coating levels, e.
g. 10 to 20mg/cm2 is able to protect drugs during long-term
storage against humidity. Even hygroscopic drugs or drugs
sensitive to hydrolysis can be coated with this aqueous
dispersion by using a reduced spraying rate at the beginning of
the coating process.
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
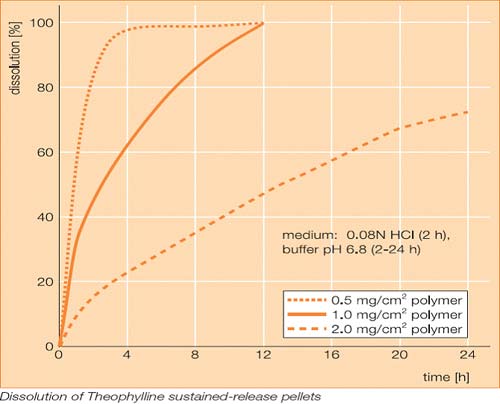
Kollicoat®SR 30D
|

|
|
|
Kollicoat ®SR 30 D is a polyvinyl acetate
dispersion stabilized with 2.7% povidone and 0.3%sodium lauryl
sulfate, with a sol ids con tent of 30%. The dispersion is suit
able for the manufacture of pH independent sustained- release
formulations. The dispersion can also be used for taste
masking.
Kollicoat ®SR 30 D is miscible with water in any ratio while
retaining its milky-white appearance. Mixing the product with
ethanol or isopropyl alcohol in a 1 : 5 ratio produces a slightly
turbid and some what viscous solution. Kollicoat ®SR 30 D is
insoluble in dilute alkaline or acidic solutions.
Sustained-
release coated formulations
It is used mainly for the manufacture of sustained- release
dosage forms. Very effective control of drug release is achieved
by coating pellets, granules and crystals.
Protective
coats
Applied in small quantities or with hydrophilic additives, it
provides good protection against odour or taste. It can also be
used, for example as a subcoating, for isolating active
ingredients to pre vent inters actions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|

|
|
|
|
|
|
Kollicoat® MAE 100P
|

|
|
|
Kollicoat® MAE 100 P is a white, redispersable
powder, originating from the Kollicoat® MAE 30 DP dispersion.
Prior to spray drying the dispersion is partially neutralized
with NaOH. This eases redispersion for the final formulation of
the spray suspension. The product consists of 95.8% copolymer,
max. 2.3 % Polysorbate 80 and max. 0.7% sodium lauryl sulfate.
Kollicoat® MAE provides an effective barrier to
gastric juice which:
- Protects the stomach
from drugs such as indomethacin, diclofenac or aspirin;
- Protects
acid-sensitive drugs,e. g. pancreatin from the contents of
the stomach,
- Increases the
bioavailability of active ingredients so that high local
drug concentrations are achieved in the small intestine
increasing the bioavailability or
- Releases the active
ingredient,e. g. laxatives, at its site of action, the
intestine.
|
|
|
|
|
|
|
|
|
|
|
|
back to top
|
|
|
|
|
|
|
|
|
|
|